`=>` The electronic configuration of oxygen atom is `1s^2 2s^2 2p^4`.
`=>` Each oxygen atom has `8` electrons, hence, in `O_2` molecule there are `16` electrons.
● The electronic configuration of `O_2` molecule, therefore, is
`O_2 : (sigma 1 s)^2 ( sigma** 1 s)^2 ( sigma 2s)^2 (sigma** 2s)^2 (sigma 2p_z)^2 (pi 2p_x^2 equiv pi 2p_y^2) (pi** 2p_x^2 equiv pi** 2p_y^2)`
or `O_2 : (KK) (sigma 2s)^2 (sigma** 2s)^2 (sigma 2p_z)^2 (pi 2p_x^2 equiv pi 2p_y^2) (pi** 2p_x^2 equiv pi** 2p_y^2)`
● From the electronic configuration of `O_2` molecule, there are ten electrons in bonding molecular orbitals and six electrons in antibonding molecular orbitals.
`=>` Bond order `= (N_b - N_a)/2 = (10-6)/2 = 2`
● So in oxygen molecule, atoms are held by a double bond.
`=>` It contains two unpaired electrons in `π **2p_x` and `π **2p_y` molecular orbitals, therefore, `O_2` molecule should be paramagnetic, a prediction that corresponds to experimental observation.
● In this way, the theory successfully explains the paramagnetic nature of oxygen.
`=>` Similarly, the electronic configurations of other homonuclear diatomic molecules of the second row of the periodic table can be written.
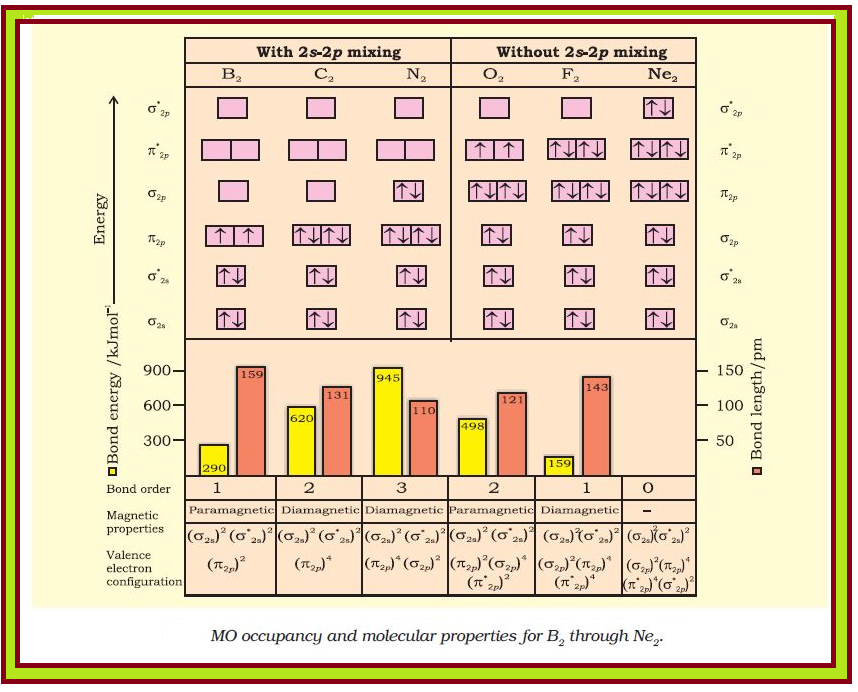
`=>` In Fig.4.21 are given the molecular orbital occupancy and molecular properties for `B_2` through `Ne_2`.
● The sequence of MOs and their electron population are shown.
● The bond energy, bond length, bond order, magnetic properties and valence electron configuration appear below the orbital diagrams.
`=>` The electronic configuration of oxygen atom is `1s^2 2s^2 2p^4`.
`=>` Each oxygen atom has `8` electrons, hence, in `O_2` molecule there are `16` electrons.
● The electronic configuration of `O_2` molecule, therefore, is
`O_2 : (sigma 1 s)^2 ( sigma** 1 s)^2 ( sigma 2s)^2 (sigma** 2s)^2 (sigma 2p_z)^2 (pi 2p_x^2 equiv pi 2p_y^2) (pi** 2p_x^2 equiv pi** 2p_y^2)`
or `O_2 : (KK) (sigma 2s)^2 (sigma** 2s)^2 (sigma 2p_z)^2 (pi 2p_x^2 equiv pi 2p_y^2) (pi** 2p_x^2 equiv pi** 2p_y^2)`
● From the electronic configuration of `O_2` molecule, there are ten electrons in bonding molecular orbitals and six electrons in antibonding molecular orbitals.
`=>` Bond order `= (N_b - N_a)/2 = (10-6)/2 = 2`
● So in oxygen molecule, atoms are held by a double bond.
`=>` It contains two unpaired electrons in `π **2p_x` and `π **2p_y` molecular orbitals, therefore, `O_2` molecule should be paramagnetic, a prediction that corresponds to experimental observation.
● In this way, the theory successfully explains the paramagnetic nature of oxygen.
`=>` Similarly, the electronic configurations of other homonuclear diatomic molecules of the second row of the periodic table can be written.
`=>` In Fig.4.21 are given the molecular orbital occupancy and molecular properties for `B_2` through `Ne_2`.
● The sequence of MOs and their electron population are shown.
● The bond energy, bond length, bond order, magnetic properties and valence electron configuration appear below the orbital diagrams.